
Often known as protein immunoblotting, the western blot method allows researchers to determine the presence, size and quantity of particular proteins in a given sample. In this blog, our team at HHC will help you explore the western blot technique to understand the procedure and its uses for protein analysis What is western blotting?ĭeveloped by Harry Towbin and his colleagues in 1979, the western blot procedure is a typical cell and molecular biology procedure widely used in the analysis of proteins. Given the diverse nature of this vital biological element, there are three traditional techniques that researchers and scientists use for protein analysis: There are different types of proteins in terms of their size and the arrangement of amino acids, having diverse molecular structures, nutritional characteristics, and chemical properties.
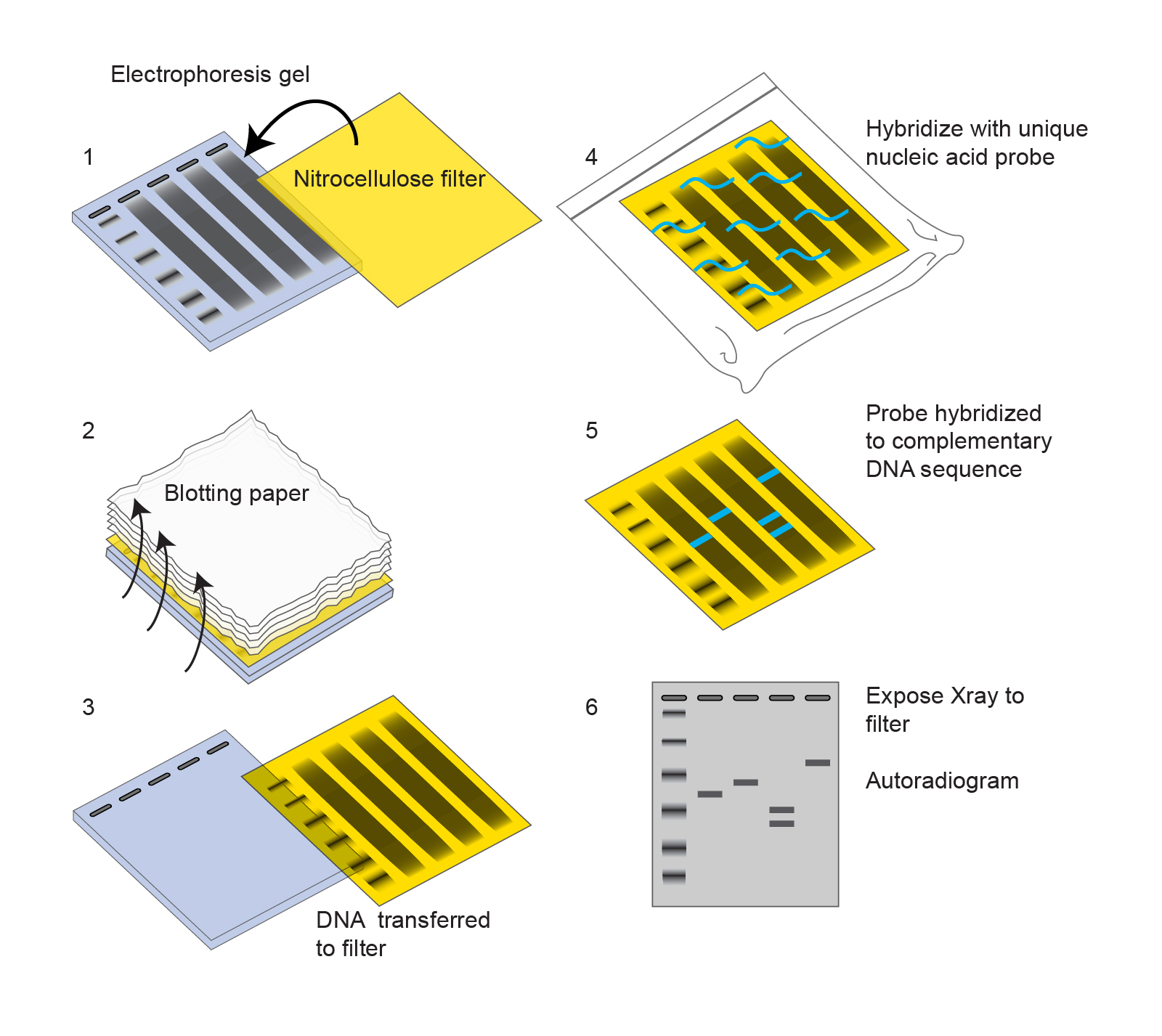
evaluating whether genetic manipulation experiments were successful or not.detecting the presence of allergenic protein in food samples and.For these reasons, detecting the presence or absence of protein, their size or molecular weight is vital. Proteins are integral to organisms, helping almost every cell process and are also critical to metabolism. Essentially proteins or polypeptides are complex compounds of amino acids that are needed by our bodies to regulate, function and construct organs and tissues in the body. This video demonstrates SDS-PAGE separation of proteins using the Bio-Rad Comparative Proteomics Kit II: Western Blot Module.Īssembly of the blotting sandwich and electroblotting are shown along with the steps for protein detection using a colorimetric assay.Proteins play many vital roles in our bodies. Western Blot Video: SDS-PAGE Separation of Proteins Please contact Bio-Rad’s Technical Services Department to learn about recommended secondary reagents for specific applications. It may be useful to include a sample in which no primary antibody is used at all, in order to determine any nonspecific binding of the secondary reagent to the target tissue. Wash the membrane with gentle agitation as follows: 4x 5 min in wash buffer 3x 5 min in PBST and 2x 5 min in PBS.Īdd appropriate enzyme substrate solution and incubate as recommended by the manufacturer to visualize protein bands.Īppropriate controls should always be carried out. Wash the blot extensively in wash buffer (3 x 10 min) with gentle agitation.Īdd appropriate enzyme-conjugated secondary antibody diluted in wash buffer and incubate for 1 hr at RT with gentle agitation. Incubate for 2 hr at RT, or overnight at 4☌.

Rinse the blot briefly with wash buffer and then add primary antibody diluted in the wash buffer (a concentration of 1-10 µg/ml is generally acceptable, but check datasheets for precise recommendations). Place blot into blocking solution for 2 hr at RT, or overnight at 4☌. PBS Disodium potassium phosphate, 1.15g Distilled water, 1 L Potassium chloride, 0.2 g Potassium dihydrogen phosphate, 0.2g Sodium chloride, 8.0 g PBST Disodium potassium phosphate, 1.15 g Distilled water, 1 L Potassium chloride, 0.2 g Potassium dihydrogen phosphate, 0.2g Sodium chloride, 8.0 gįollowing SDS-PAGE, transfer proteins onto blotting membrane according to the manufacturer’s instructions.Ĭheck protein transfer by staining the blot with Ponceau S for 1 min, then completely destain the blot by washing with distilled water. Washing buffer Blocking buffer + 0.1% Tween 20 Ponceau S Acetic acid, 5 ml However, we advise using our protocol for detection of phosphorylated proteins by western blot. *Note: For cleaner western blots, Block Ace is recommended over 5% non-fat dried milk dissolved in PBS. For the detection of phosphorylated protein, use the recommended blocking solution as stated on the product datasheet. Blocking Buffer Block Ace BUF029 dissolved in water, or 5% non-fat dried milk dissolved in PBS.


 0 kommentar(er)
0 kommentar(er)
